Advancing quantum chemistry in pharmaceuticals & agriculture with Boehringer Ingelheim
Boehringer Ingelheim and PsiQuantum’s new research brings closer to reality the ability to calculate the electronic structure of the heme group of Cytochrome P450 and of FeMoco on a quantum computer by finding a speedup of the calculation by a factor of 234x and 278x, respectively, representing the new state-of-the-art estimates for both systems
Key Insights
Cytochrome P450 and FeMoco are two particularly interesting chemical systems for the application of quantum computing given their computational complexity.
Boehringer Ingelheim and PsiQuantum’s new research brings closer to reality the ability to calculate the electronic structure of the heme group of Cytochrome P450 and of FeMoco on a quantum computer by finding a speedup of the calculation by a factor of 234x and 278x, respectively, representing the new state-of-the-art estimates for both systems.
The factors of 234x and 278x have three contributions:
Active Volume (AV) architecture: 25.2x and 31.4x
Combination of Block-Invariant Symmetry Shift (BLISS) and Tensor Hypercontraction (THC) techniques: 8.2x and 8.1x
Circuit optimization: 1.1x (10%), for both systems
This result would not be possible without PsiQuantum’s unique AV-compatible hardware architecture and fast gate execution time that other hardware cannot easily replicate.
Moreover, Boehringer Ingelheim and PsiQuantum now have the knowledge of how to combine two cutting-edge methods, BLISS and THC, and have set their sights on developing further methods to speed up the calculations.
Context setting: Cytochrome P450 and FeMoco, and its real-world applications
Quantum computers are heralded as the next generation of computational tools for complex quantum-chemistry calculations. Researchers worldwide are working hard to identify chemical systems where a quantum computer could unlock key insights into their behavior, and this work focuses on two systems where better calculations would enable progress in the pharmaceutical and chemical industry.
The first one is the heme group in a group of enzymes known as Cytochrome P450, which are essential for the absorption and metabolization of more than 60% of drugs in the human body. Improved understanding of their properties through quick and reliable calculations would allow pharmaceutical researchers to improve the way that they design medication, and ultimately improve drug effectiveness. The second one is the iron-molybdenum cofactor (FeMoco) in nitrogenase, a key enzyme in the process of nitrogen fixation, the conversion of atmospheric nitrogen (N2) into ammonia (NH3) needed for plant growth. Understanding the properties and functionality of FeMoco would allow us to develop less energy-intensive fertilizers based on it that preserve the level of food production, while using dramatically less energy.
Novel research in preparing the calculation
Chemical properties of a quantum system are calculated from the energy eigenvalues of the system’s Hamiltonian, which is where the quantum computer would step in; however, brute-forcing the issue would be very inefficient. Therefore, methods have been developed to reduce the size of the calculation problem before it is even loaded into the quantum computer: Tensor Hypercontraction (THC) and Block-Invariant Symmetry Shift (BLISS). These methods have previously been used separately, but this research represents the first instance of their combination. Combining THC and BLISS results in a >8x speed up for both Cytochrome P450 and FeMoco.
THC exploits the sparsity of the 4-dimensional 2-electron-integral tensor in the Hamiltonian and replaces it with a product of 2-dimensional matrices, thereby significantly reducing the number of qubits required to represent the system.
BLISS adds to that by shifting the entire Hamiltonian by a well-behaved symmetry function that preserves the relations between the eigenvalues in the sector of interest for the computation but reduces the overall 1-norm by shifting the eigenvalues in other sectors.
Essential Terms:
-
A Hamiltonian represents the total energy of the quantum system based on the contributions from the system’s constituent components. Finding the quantized energy levels of the quantum system represents finding the eigenvalues of the Hamiltonian. This is done by solving the equation of the system, most often the Schrödinger equation. Solving the equation also finds the characteristic states of the quantum system – the eigenstates – which in molecules represent electronic configurations
-
Energy contribution to the system’s Hamiltonian coming from a single electron’s kinetic energy and its electric interaction with the core of the atom
-
Energy contribution to the system’s Hamiltonian coming from the interactions between two electrons – electric repulsion and the exchange interaction
-
The 1-norm λ is a scaling parameter used in the encoding of the eigenvalues of the Hamiltonian when preparing the calculation on the quantum computer
-
the number of qubits and the number of operations required for a quantum algorithm to be executed; the goal of research is to reduce these as much as possible to enable the execution of the quantum algorithm on smaller quantum hardware that is available earlier
Novel quantum architecture: Active Volume
This research also presents the first estimate of quantum resources for the Cytochrome P450 and FeMoco problems using an Active Volume (AV) architecture. As its name suggests, an AV architecture executes active operations in a logical circuit, such as Clifford and Toffoli gates, without wasting resources in executing idle operations. On the other hand, in a generic, unoptimized quantum architecture, one part of the calculation has to wait for the result of another part of the calculation, which also includes idle operations, to finish before it can continue. Moreover, with additional connections between qubits, an AV quantum computer can maximize the instance size that is addressable on the device by executing the active operations in parallel. In this concrete case, this improvement leads to a speedup of the calculations by a factor of 25.2x and 31.4x for Cytochrome P450 and FeMoco, respectively.
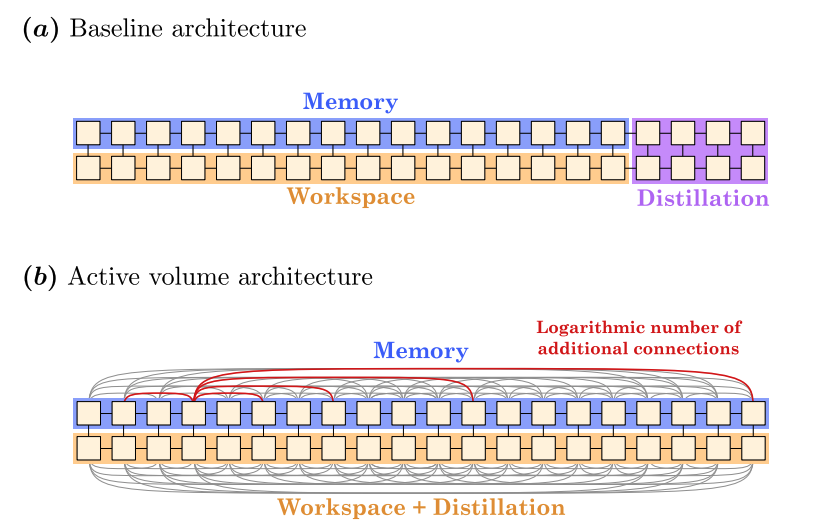
Figure 1: Active Volume Architecture
Schematic representation of the difference between the Baseline architecture and the Active Volume architecture for logical qubits (yellow squares). (a) The Baseline architecture is a 2D architecture in which logical qubits are connected to their nearest neighbors. It is split into three sections: (i) the Memory section stores the information that is used in the quantum algorithm, (ii) the Workspace section is used to connect the different logical qubits from the Memory section when operations are required between them, and (iii) the Distillation section produces the magic T states required for quantum operations via magic-state distillation. (b) The Active Volume architecture introduces additional connections between logical qubits. Additionally, the Distillation section is absorbed into the Workspace section to increase the utilization of the logical qubits. Here, the additional connections for the 4th logical qubit from the left in the Memory section are represented.
What’s next?
While significant, this result is not the final step in speeding up calculations. There are further improvements that can be made in the preparation of the Hamiltonian before we run into theoretical limits, e.g., the 1-norm of the Hamiltonian is still ~2x higher than the limit for the Cytochrome P450 case. Moreover, the AV architecture can bear further scrutiny because the current result assumes upper bounds on subroutines in the algorithm, representing worst-case estimates, meaning that a tighter analysis of AV costs would have an immediate impact on the speedup without changes to the algorithm itself. Working on these and other possible improvements is the next target, and Boehringer Ingelheim and PsiQuantum look forward to the collaboration that will enable these improvements.